I tend to think so, at least for me. I had always taken at face value the normal statement about if you take power out of a battery faster, you get less total power out of it. I think I was just assuming the chemical reaction couldn't keep up with the rate at high discharges, so you were out of reaction earlier. Today, with all the talk of big alternators, large banks, fast discharges, etc, I started to wonder about what happens if you draw down the first half of battery fast and the second half slow. Do you still come up short because of the fast first half? According to my chemical reaction idea it would be likely the battery would recover a bit as the chemistry got a chance to stabilize at the lower output, so you might get the capacity back.
When I went looking for information, I remembered that Bogart Engineering had an explanation of why they don't use Peukert, so I tracked that down. It was very interesting to me and it blew up just about everything I has had assumed.
Bottom line, according to Bogart, is that the battery will always be able to give up exactly the same amount of power, regardless of discharge rate, as long as the end of the discharge is at a low rate. The explanation is so simple it is borderline silly.
http://bogartengineering.com/sites/default/files/docs/PeukertsComments.pdf
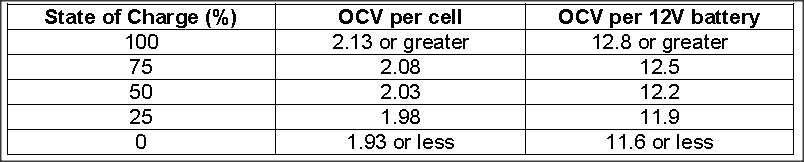
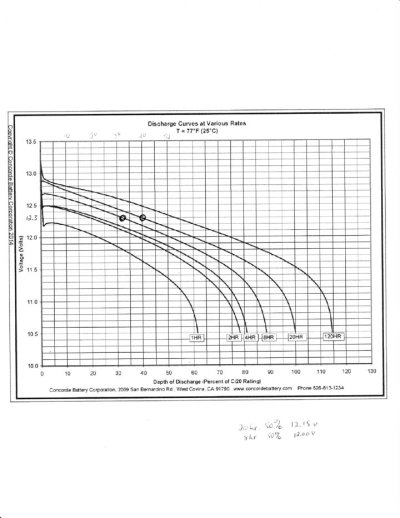
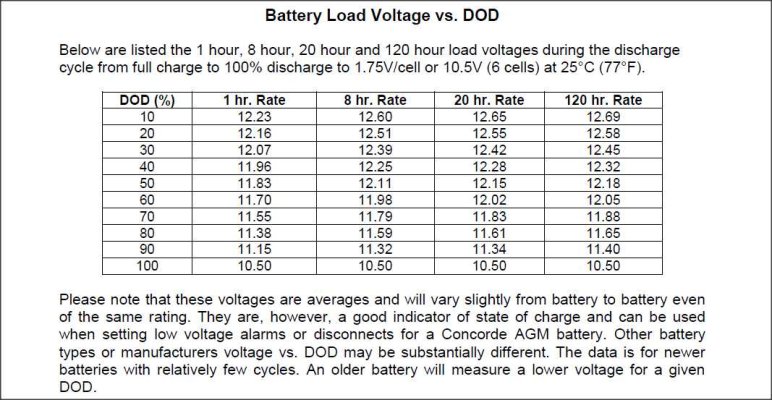
The only reason a battery gives up less power at a high discharge rate is because the definition of discharged is getting to 10.5 volts under the load being tested and the bigger the load, the more voltage is dropped because of that load. The battery isn't less full near the end of fast discharge, it just has more voltage drop during the entire discharge than if it was slow, so it reaches 10.5v easier. It actually be more full than one drained slowly.
This would totally explain why AGMs have much lower Peukert coefficient than wet cells. It is only because they drop less voltage under load, which has nothing to do with capacity at all. It is often said that lithium does not have a Peukert effect, which makes sense because they give up power even easier and drop even less voltage under load.
I think I now have a much better idea of how it works in the real world, and that way is to just ignore it. I had wondered if we ran the batteries to 50% of rated AH running something that was a big load, how much would we really have left? I had assumed that it would be under 50% of AH, but that is not right. It will be just as it appears. Just go be the number of AH you have used to figure where you are in discharge, and you will be correct no matter how you discharged the batteries.
Nice to know, at least for me
When I went looking for information, I remembered that Bogart Engineering had an explanation of why they don't use Peukert, so I tracked that down. It was very interesting to me and it blew up just about everything I has had assumed.
Bottom line, according to Bogart, is that the battery will always be able to give up exactly the same amount of power, regardless of discharge rate, as long as the end of the discharge is at a low rate. The explanation is so simple it is borderline silly.
http://bogartengineering.com/sites/default/files/docs/PeukertsComments.pdf
The only reason a battery gives up less power at a high discharge rate is because the definition of discharged is getting to 10.5 volts under the load being tested and the bigger the load, the more voltage is dropped because of that load. The battery isn't less full near the end of fast discharge, it just has more voltage drop during the entire discharge than if it was slow, so it reaches 10.5v easier. It actually be more full than one drained slowly.
This would totally explain why AGMs have much lower Peukert coefficient than wet cells. It is only because they drop less voltage under load, which has nothing to do with capacity at all. It is often said that lithium does not have a Peukert effect, which makes sense because they give up power even easier and drop even less voltage under load.
I think I now have a much better idea of how it works in the real world, and that way is to just ignore it. I had wondered if we ran the batteries to 50% of rated AH running something that was a big load, how much would we really have left? I had assumed that it would be under 50% of AH, but that is not right. It will be just as it appears. Just go be the number of AH you have used to figure where you are in discharge, and you will be correct no matter how you discharged the batteries.
Nice to know, at least for me
Last edited: